Days: 2 7:30 AM- 4:30 PM MST Learn Pharma & Medical Device CAPA Come learn the FDA 21 CFR and ISO 13485 requirements for a regulatory-...
Read MoreThis content is password protected. To view it please enter your password below: Password:
Read MoreDays: 4 • CTUs: 3.6 Monday - Thursday 7:30am - 5:30pm MST 5 Day Schedule: 7:30 AM – 5:30 PM MST Audience: Beginner t...
Read More5-Day AS9100 Rev D and ISO 9001:2015 Lead Auditor Training Course 5 Day Schedule: 7:30 AM – 6:30 PM MST Mon - Thu ...
Read More4 Day Schedule: 7:30 AM – 5:30 PM MST Audience: Beginner to Advanced Axeon is an Exemplar Glo...
Read MoreOur AS9110 Lead Auditor Training will teach you how to use auditing to improve your organization 5 Day Schedule: 7:30 AM ...
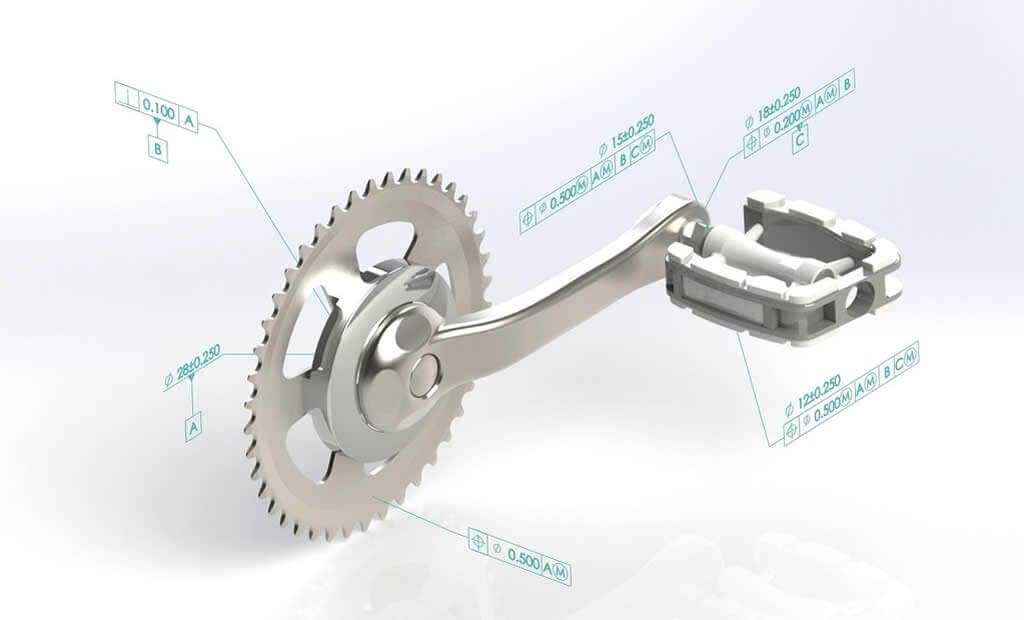
Read MoreDays: 3 7:30 AM- 4:30 PM MST Overview This intermediate course begins as an introduction to the ASME Y14.5 standard and the history of G...
Read MoreDays: 3 7:30 AM- 4:30 PM MST Overview The AS13100 AESQ (Aerospace Engine Supplier Quality) Supplemental QMS Requirements simplifies and ...
Read MoreAdvanced Product Quality Planning and Production Part Approval Process- APQP and PPAP Training Days: 2 Time: 7:30 AM – 4:30 PM MST Overvie...
Read MoreDays: 2 Time: 7:30 AM- 4:30 PM MST Axeon's training courses begin with your objectives in mind. Before any training begins the instructor mus...
Read More